Background: Flumatinib mesylate, as a novel oral BCR-ABL1 tyrosine kinase inhibitor, is approved as a frontline therapy for chronic myeloid leukemia in chronic phase (CML-CP) in China since 2019. Mechanistically, with its structure of trifluoromethyl and pyridine flumatinib blocked BCR-ABL1 kinase autophosphorylation with much more potent activity than did imatinib and even showed higher efficacy than nilotinib against wild-type BCR-ABL1 kinase. Currently, there is still lacking the real-world effectiveness and safety of flumatinib in patients with CML.
Methods: We retrospectively analyzed efficacy and safety of CML-CP patients (≥16 years) used flumatinib (600mg daily) or imatinib (400mg daily) as first-line treatment from 2020 to 2023, presenting a real-world comparison study. A propensity score (PS) matching was performed to adjust for Sokal risk score to compare the treatment efficacy, survival outcomes and safety of flumatinib and imatinib in a clinically well matched cohort.
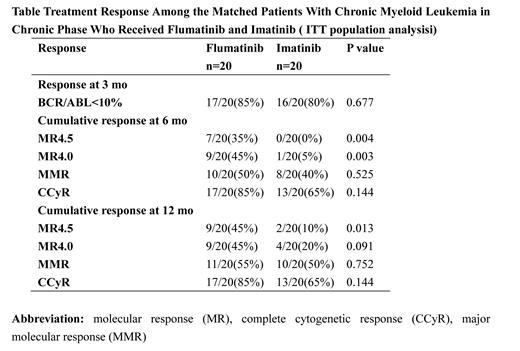
Results: PS matching resulted in 20 patients from each cohort being matched for Sokal risk scores. The median follow-up of the flumatinib-treated and imatinib-treated cohorts was 441 days and 487 days, respectively. In the intention-to-treat (ITT) population analysis, there is no differences between flumatinib and imatinib groups in 3-month early molecular response rate (85% vs. 80%, p = 0.677), 6-month major molecular response rate (MMR) (50% vs. 40%, p = 0.525) and 12-month MMR (55% vs. 50%, p = 0.752). In term of deep molecular response, more patients receiving flumatinib achieved molecular remission 4.5 (MR4.5) and molecular remission 4 (MR4.0) at 6 months (35% vs.0%, p = 0.004; and 45% vs. 5.0%, p = 0.003, respectively) and appeared to have higher 12-month MR4.5 and 12-month MR4.0 (45% vs.10%, p = 0.013; and 45% vs. 20%, p = 0.091, respectively; Table). During the follow-up period, treatment discontinuation occurred in 6 and 9 patients in flumatinib-treated and imatinib-treated cohorts respectively, including 2 and 3 patients due to drug resistance and 3 and 4 patients due to toxicity. The 2-year probability of event-free survival was 70% among the patients who received flumatinib and 47% among those who received imatinib ( p = 0.85), and the corresponding 2-year failure-free survival probabilities were 85% and 76%, respectively ( p = 0.77). Adverse events of edema, rash were more frequent in imatinib arm (any grade; 0% vs. 25%, p = 0.017; and 0% vs. 25%, p = 0.035, respectively), whereas alanine transaminase elevation appeared to be more frequent in flumatinib arm (any grade, 15% vs. 0%, p = 0.072). Of note, one patient receiving flumatinib had grade 3 QT prolongation and ventricular premature beat and was discontinued.
Conclusions: The real-world data demonstrated that as a first-line treatment setting, flumatinib can bring patients with chronic phase CML higher rates of responses, and faster and deeper responses, indicating that flumatinib could be an alternative effective first-line treatment for CML-CP. The adverse events of flumatinib, such as cardiovascular events, abnormal liver function, and diarrhea, need to be given continuous concern in future studies.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal